If you've suffered serious side effects from diabetes medications like Ozempic, Mounjaro, Wegovy, or Trulicity, you may be entitled to compensation. Thousands of patients are filing lawsuits against manufacturers Eli Lilly and Novo Nordisk for failing to warn about severe gastrointestinal injuries, vision loss, and other life-threatening complications caused by GLP-1 drugs.
Free Case Evaluation | No Fees Unless We Win | Bilingual Services Available (中文)
What Are GLP-1 Diabetes Lawsuits?
GLP-1 diabetes lawsuits seek compensation for patients who suffered severe injuries from glucagon-like peptide-1 receptor agonist (GLP-1 RA) medications. These drugs, marketed for type 2 diabetes treatment and weight loss, have been linked to serious complications that manufacturers allegedly failed to disclose.
Lawsuits are currently being consolidated into multidistrict litigation (MDL) in the U.S. District Court for the Eastern District of Pennsylvania, streamlining the legal process for injured patients nationwide.
Active Diabetes Drug Lawsuits
GLP-1 Medication Lawsuits
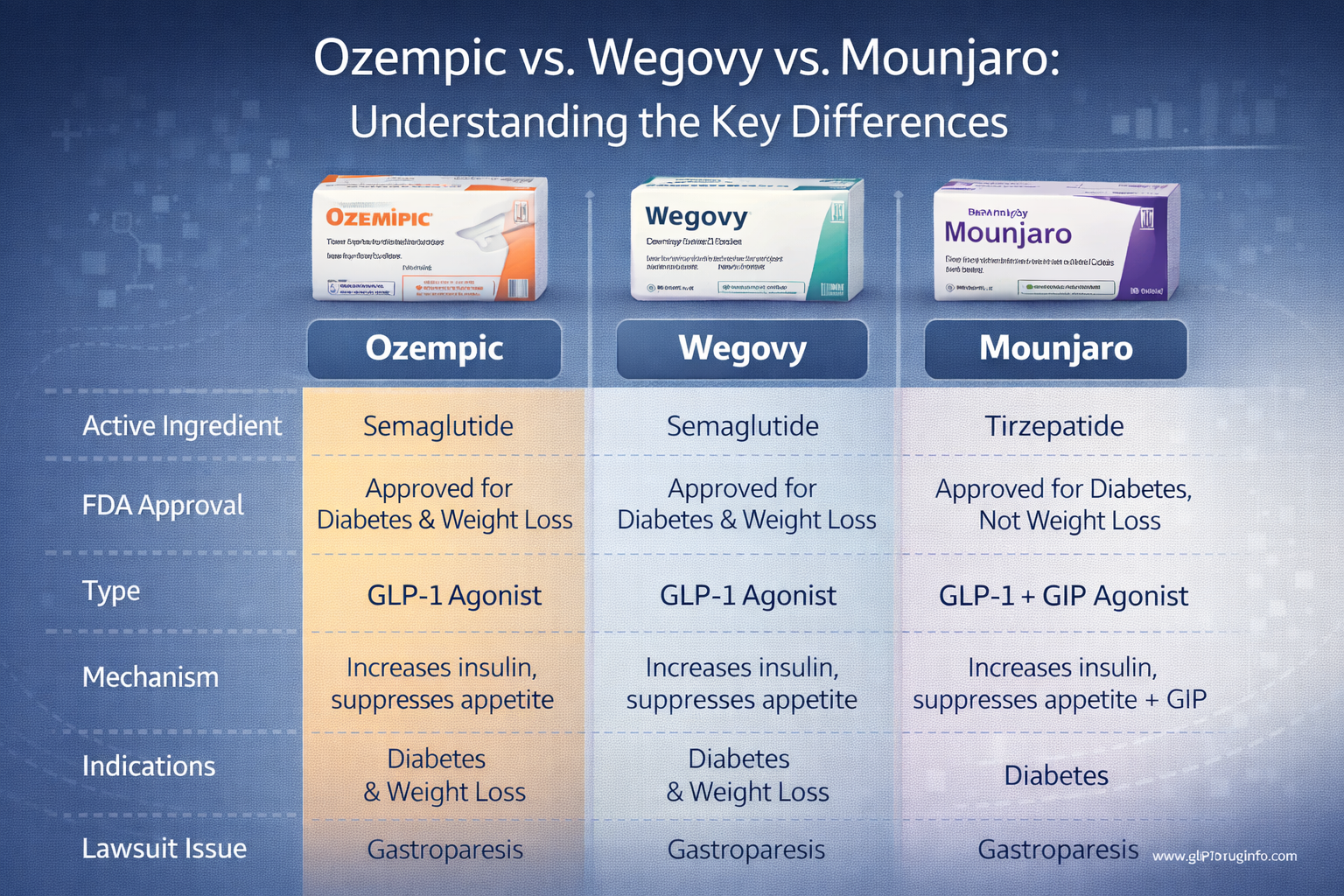
- Ozempic Lawsuit - Semaglutide injection for diabetes
- Wegovy Lawsuit - Semaglutide injection for weight loss
- Rybelsus Lawsuit - Oral semaglutide tablet
- Mounjaro Lawsuit - Tirzepatide injection for diabetes
- Zepbound Lawsuit - Tirzepatide injection for weight loss
- Trulicity Lawsuit - Dulaglutide injection for diabetes
- Saxenda Lawsuit - Liraglutide injection for weight loss
- Victoza Lawsuit - Liraglutide injection for diabetes
How Do GLP-1 Drugs Work?
GLP-1 receptor agonists mimic a naturally occurring hormone in your body that regulates blood sugar and appetite. These medications:
- Stimulate insulin release from the pancreas
- Suppress glucagon secretion (which raises blood sugar)
- Slow gastric emptying (digestion)
- Increase feelings of fullness after eating
The FDA approved the first GLP-1 drug for type 2 diabetes in 2005. Since then, these medications have become increasingly popular for both diabetes management and weight loss. However, many physicians prescribe diabetes-approved GLP-1 drugs off-label for weight loss, expanding their use beyond FDA-approved indications.
Popular GLP-1 Brand Names
Diabetes Treatment:
- Ozempic (semaglutide)
- Mounjaro (tirzepatide)
- Trulicity (dulaglutide)
- Victoza (liraglutide)
- Rybelsus (oral semaglutide)
Weight Loss Treatment:
- Wegovy (semaglutide)
- Zepbound (tirzepatide)
- Saxenda (liraglutide)
Serious Side Effects Linked to GLP-1 Drugs
Patients taking GLP-1 medications have reported severe, sometimes life-threatening complications that were not adequately disclosed on warning labels:
Gastrointestinal Injuries
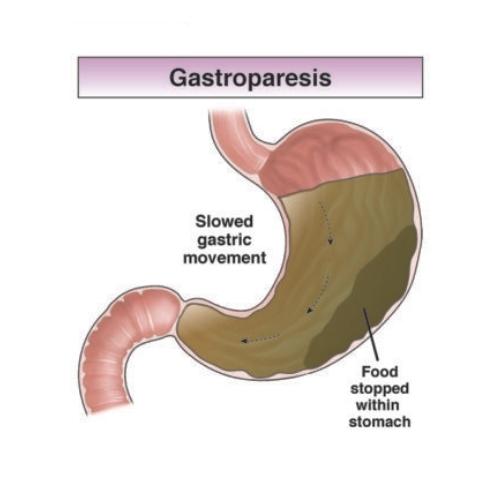
- Gastroparesis (stomach paralysis) - permanent inability to digest food normally
- Intestinal obstruction or blockage - requires emergency surgery
- Ileus - bowel cannot move waste through the body
- Gastroenteritis - severe inflammation of stomach and intestines
- Esophageal injury - damage to the esophagus
Other Serious Complications
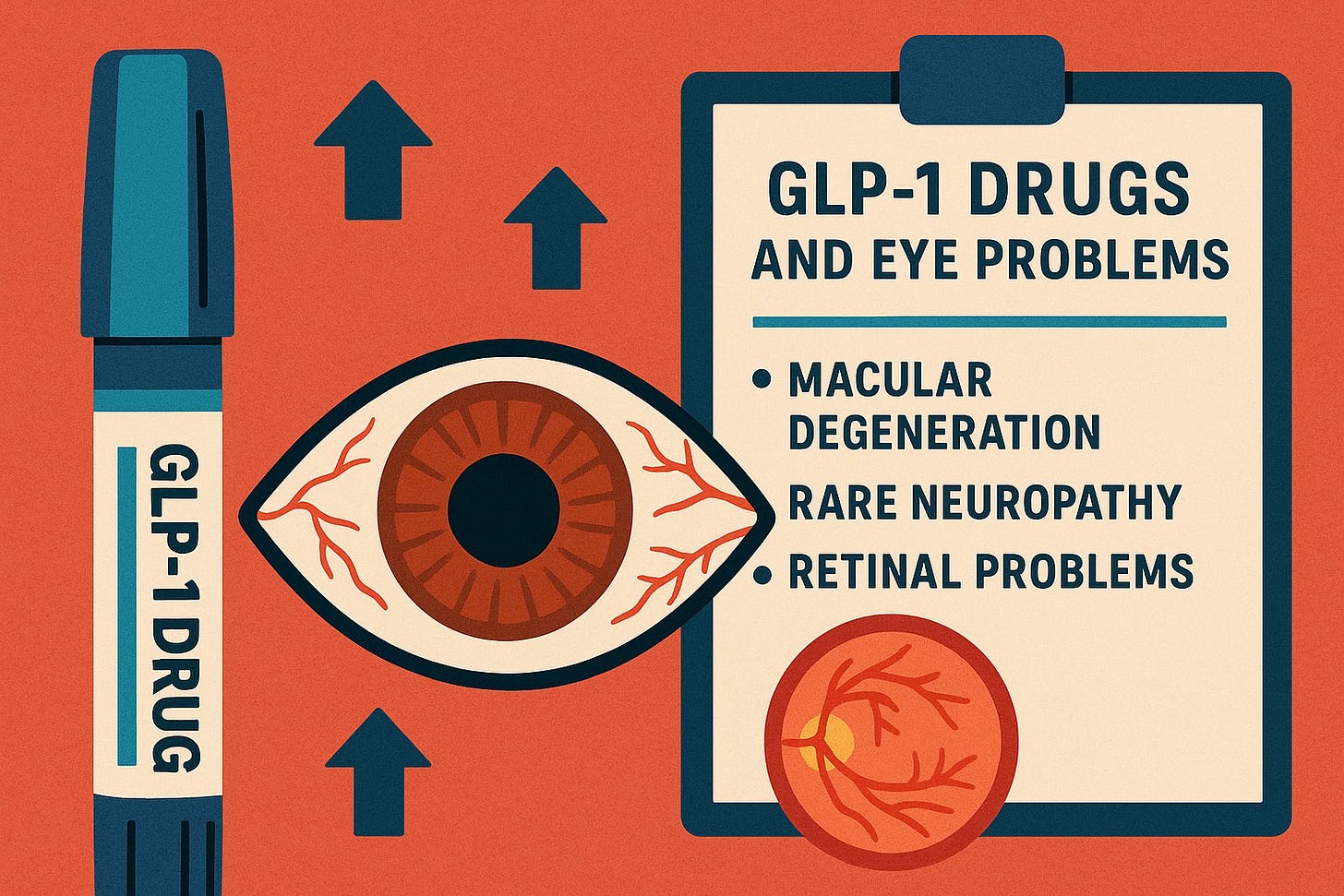
- Vision loss and blindness - from diabetic retinopathy
- Deep vein thrombosis (DVT) - dangerous blood clots
- Aspiration during surgery - inhaling stomach contents during procedures
- Malnutrition - from chronic digestive problems
- Gallbladder disease - requiring removal
- Pancreatitis - painful inflammation of the pancreas
- Thyroid tumors - including thyroid cancer
- Acute kidney injury
Many of these side effects were not included on medication labels when patients were prescribed these drugs, depriving them of informed consent.
Why Are People Suing GLP-1 Manufacturers?
Lawsuits against Eli Lilly and Novo Nordisk allege:
- Failure to warn - Manufacturers knew or should have known about severe gastrointestinal risks but did not adequately warn patients or doctors
- Defective design - The drugs are unreasonably dangerous for their intended use
- Negligence - Companies prioritized profits over patient safety
- Fraudulent concealment - Manufacturers hid evidence of serious side effects
Patients have the legal right to be fully informed about medication risks before taking them. When pharmaceutical companies withhold critical safety information, they can be held liable for resulting injuries.
Compensation Available in GLP-1 Lawsuits
If you suffered injuries from a GLP-1 medication, you may recover compensation for:
- Medical expenses - past and future treatment costs
- Lost wages - time missed from work
- Loss of earning capacity - if you can no longer work
- Pain and suffering - physical discomfort and emotional distress
- Diminished quality of life - permanent lifestyle limitations
- Punitive damages - in cases of egregious corporate misconduct
Individual GLP-1 Drug Lawsuits
Ozempic Lawsuits (Semaglutide)
Ozempic, manufactured by Novo Nordisk, is a once-weekly injectable approved for type 2 diabetes in December 2017. While not FDA-approved for weight loss, off-label prescribing has exploded. Prescriptions increased from 2 million in 2019 to 8.2 million in 2021.
Reported injuries: Gastroparesis, intestinal blockages, DVT, vision loss, severe vomiting, malnutrition
Patients are suing Novo Nordisk for failing to warn about gastrointestinal paralysis and other severe complications. Studies have also linked semaglutide to non-arteritic anterior ischemic optic neuropathy (NAION), a form of sudden vision loss.
Learn more about Ozempic lawsuits
Wegovy Lawsuits (Semaglutide)
Wegovy is Novo Nordisk's higher-dose version of semaglutide, FDA-approved specifically for chronic weight management in June 2021. It was approved for teens 12+ in December 2022 and for cardiovascular risk reduction in March 2024.
Novo Nordisk has spent over $25 million marketing Wegovy to medical professionals in the past decade. Sales increased by more than 1,361% between July 2021 and December 2023.
Reported injuries: Gastroparesis, bowel obstruction, severe dehydration, gallbladder disease
Learn more about Wegovy lawsuits
Rybelsus Lawsuits (Oral Semaglutide)
Rybelsus is the first oral GLP-1 medication for type 2 diabetes, approved by the FDA in 2019. Patients take it daily, 30 minutes before eating or drinking.
Despite being a different delivery method, Rybelsus contains the same active ingredient (semaglutide) as Ozempic and Wegovy and has been linked to similar complications.
Novo Nordisk's warnings about Rybelsus side effects demonstrate the company knew semaglutide could cause serious harm, yet continued aggressively marketing other semaglutide products without adequate gastroparesis warnings.
Learn more about Rybelsus lawsuits
Mounjaro Lawsuits (Tirzepatide)
Mounjaro, manufactured by Eli Lilly, contains the active ingredient tirzepatide. It was FDA-approved for type 2 diabetes treatment in May 2022 and is administered as a once-weekly injection.
Label warnings include: Thyroid tumors, pancreatitis, gallbladder disease, diabetic retinopathy (vision loss)
Unlabeled complications reported by patients: DVT, gastroparesis, ileus, bowel perforation, intestinal obstruction
Lawsuits allege Eli Lilly knew about severe gastrointestinal risks but failed to adequately warn patients and prescribers.
Learn more about Mounjaro lawsuits
Zepbound Lawsuits (Tirzepatide)
Zepbound is Eli Lilly's brand name for tirzepatide marketed specifically for weight loss. It received FDA approval in November 2023—making it one of the newest GLP-1 medications on the market.
Zepbound is essentially identical to Mounjaro in composition, administration, and dosage. In August 2024, Eli Lilly began offering direct-to-consumer sales without insurance at reduced prices, significantly expanding access.
Zepbound is included in the federal MDL, with plaintiffs reporting the same dangerous side effects as Mounjaro users.
Learn more about Zepbound lawsuits
Trulicity Lawsuits (Dulaglutide)
Trulicity, made by Eli Lilly, contains the GLP-1 dulaglutide. FDA-approved in 2014 for diabetes blood sugar control, it was later approved in 2020 to reduce cardiovascular risk.
Despite not being approved for weight loss, Trulicity has been widely prescribed off-label, contributing to drug shortages.
Reported unlabeled side effects: Blood clots, DVT, gallbladder disease, gastroenteritis, ileus, intestinal obstruction, aspiration during surgery
Notably, Eli Lilly did not add diabetic retinopathy (vision loss) warnings to Trulicity's label until 2020, years after approval and patient reports.
Learn more about Trulicity lawsuits
Saxenda Lawsuits (Liraglutide)
Saxenda is Novo Nordisk's liraglutide-based weight loss medication, FDA-approved for adult obesity treatment in December 2014 and for teens 12-17 in December 2020.
Administered as a daily injection, Saxenda is less effective than newer semaglutide drugs (patients lost 5% body weight vs. 15% with Ozempic in trials) but carries similar risks.
Label warnings: Pancreatitis, gallbladder disease, thyroid tumors, suicidal thoughts
Unlabeled complications: Gastroparesis, chronic digestive disorders
Plaintiffs allege Novo Nordisk failed to disclose the full extent of gastrointestinal injury risks.
Learn more about Saxenda lawsuits
Victoza Lawsuits (Liraglutide)
Victoza, Saxenda's predecessor, is also manufactured by Novo Nordisk and contains liraglutide. FDA-approved for adult diabetes in 2010, children 10+ in 2019, and cardiovascular risk reduction in 2017.
Administered as a daily injection with slightly different dosing than Saxenda.
Known side effects: Kidney injury, pancreatitis, gallbladder disease, thyroid tumors
Research shows liraglutide increases gastroparesis and intestinal blockage risk, yet the manufacturer did not include adequate warnings. Victoza is part of the federal GLP-1 MDL.
Learn more about Victoza lawsuits
Medical Device Lawsuits
Dexcom G6 Glucose Monitor Lawsuits
The Dexcom G6 is an implantable continuous glucose monitoring (CGM) system that transmits blood sugar data to a smartphone app. Accurate glucose monitoring is critical for diabetic patients to manage insulin and avoid dangerous blood sugar fluctuations.
Thousands of patients have reported to the FDA:
- Inaccurate glucose readings
- Alert failures (not warning of dangerous blood sugar levels)
- System malfunctions
Faulty glucose monitoring can lead to improper insulin dosing, resulting in hypoglycemia (dangerously low blood sugar) or hyperglycemia (dangerously high blood sugar)—both potentially life-threatening.
Patients injured by defective Dexcom G6 systems are filing lawsuits against Dexcom, Inc.
MiniMed Insulin Pump Lawsuits
MiniMed insulin pumps, manufactured by Medtronic, are designed to automatically deliver programmed insulin doses to diabetic patients. However, several MiniMed models have experienced defects causing incorrect insulin delivery.
Risks of improper insulin dosing:
- Hypoglycemia (low blood sugar) - can cause confusion, seizures, loss of consciousness, death
- Hyperglycemia (high blood sugar) - can lead to diabetic ketoacidosis, organ damage, coma
Patients harmed by defective MiniMed insulin pumps are pursuing legal action against Medtronic for product liability.
Do You Qualify for a Diabetes Lawsuit?
You may be eligible to file a lawsuit if:
- You took a GLP-1 medication (Ozempic, Mounjaro, Wegovy, Trulicity, etc.)
- You suffered serious side effects such as gastroparesis, intestinal blockage, vision loss, or DVT
- Your injuries required hospitalization, surgery, or ongoing medical treatment
- You were not adequately warned about these risks before taking the medication
OR
- You used a Dexcom G6 glucose monitor or MiniMed insulin pump
- The device malfunctioned or provided inaccurate readings
- You suffered injuries as a result (hypoglycemia, hyperglycemia, hospitalization)
No Upfront Costs
Diabetes drug lawsuits are handled on a contingency fee basis, meaning:
- No fees unless we recover compensation for you
- Free case evaluation
- No out-of-pocket expenses
- We advance all litigation costs
Why Work With an Attorney Who Understands Both Sides
Successfully navigating GLP-1 litigation requires more than general personal injury experience, it demands deep knowledge of pharmaceutical regulation, FDA approval processes, and corporate decision-making at major drug companies.
Insider Pharmaceutical Industry Experience
Before representing injured patients, I worked in-house for a multi-national pharmaceutical company specializing in diabetes treatment, handling:
- Regulatory compliance with FDA requirements
- Drug approval processes
- Corporate legal matters and risk assessment
- Safety data evaluation
This insider experience gives me unique insight into:
- How pharmaceutical companies evaluate safety signals
- Corporate decision-making regarding label warnings
- Litigation defense strategies employed by drug manufacturers
- Industry standards for adequate warnings
Federal MDL Experience
Combined with over 10 years of experience in complex multidistrict litigation (MDL) in federal courts, I know how to:
- Anticipate defense arguments before they're made
- Build cases that withstand aggressive challenges from elite defense firms
- Navigate the unique procedural requirements of MDL proceedings
- Maximize efficiency while preparing for individual trials
National Resources with Personal Service
I work in partnership with one of the largest plaintiff law firms in the nation, giving you access to:
- Extensive financial resources for expert witnesses
- Medical experts specializing in gastroenterology, endocrinology, and pharmacology
- Cutting-edge litigation technology and support staff
- Coordinated national litigation strategy
But you're not just a case number. You work directly with me throughout your case, receiving personalized attention and responsive communication.
Bilingual Legal Services
We also provide legal consultations and services in Mandarin Chinese (中文) for clients who prefer to discuss their case in their native language.
How We Investigate Your Diabetes Lawsuit
If you believe a GLP-1 medication, faulty glucose monitoring system, or defective insulin pump caused your injuries, our investigation includes:
Medication/Device Review
- Identify the specific drug or device involved
- Determine FDA approval status and labeled indications
- Research known adverse event reports
Medical Causation Analysis
- Review your complete medical records
- Consult with medical experts in relevant specialties
- Establish timeline linking medication/device use to your injuries
- Rule out alternative causes
Regulatory Compliance Investigation
- Examine manufacturer's compliance with FDA regulations
- Review clinical trial data and safety studies
- Investigate adverse event reports submitted to FDA
- Analyze when manufacturers knew or should have known about risks
Warning Adequacy Assessment
- Compare medication labels over time
- Determine what warnings were provided when you were prescribed the drug
- Evaluate whether warnings were adequate under applicable legal standards
- Investigate marketing materials for misrepresentation
Liability Analysis
- Identify all potentially liable parties (manufacturers, distributors, prescribers)
- Assess strength of product liability claims
- Evaluate potential damages
- Determine optimal litigation strategy
Take Action: Free Case Evaluation
If you've suffered serious side effects from a GLP-1 diabetes or weight loss medication, or injuries from a defective glucose monitor or insulin pump, time may be limited to file your claim.
Contact me today for a free, confidential case evaluation. We'll discuss your situation honestly and determine the best path forward, with no obligation and no upfront costs.
Mandarin Chinese services available
Frequently Asked Questions
Q: How much does it cost to hire a diabetes lawsuit attorney?A: Nothing upfront. We work on contingency, meaning we only get paid if we recover compensation for you.
Q: What is the deadline to file a diabetes drug lawsuit?A: Statutes of limitations vary by state, typically 1-3 years from when you discovered your injury. Contact us immediately to protect your rights.
Q: Can I still file a lawsuit if I signed a consent form?A: Yes. Consent forms don't waive your right to sue if you weren't adequately warned about known risks.
Q: What if I'm still taking the medication?A: You may still qualify. Consult your doctor about your medication, and consult us about your legal rights.
Q: How long do diabetes drug lawsuits take?A: MDL cases typically take 2-4 years. Some cases may settle sooner; others may go to trial.
Last updated and reviewed by a licensed attorney: January 2026
Disclaimer: This website is for informational purposes only and does not constitute legal advice. No attorney-client relationship is formed by reading this content or contacting us for a consultation. Past results do not guarantee future outcomes. Each case is unique and evaluated on its individual merits.