GLP-1 Drugs and NAION: What Patients Need to Know About the Rising Vision-Loss Lawsuits
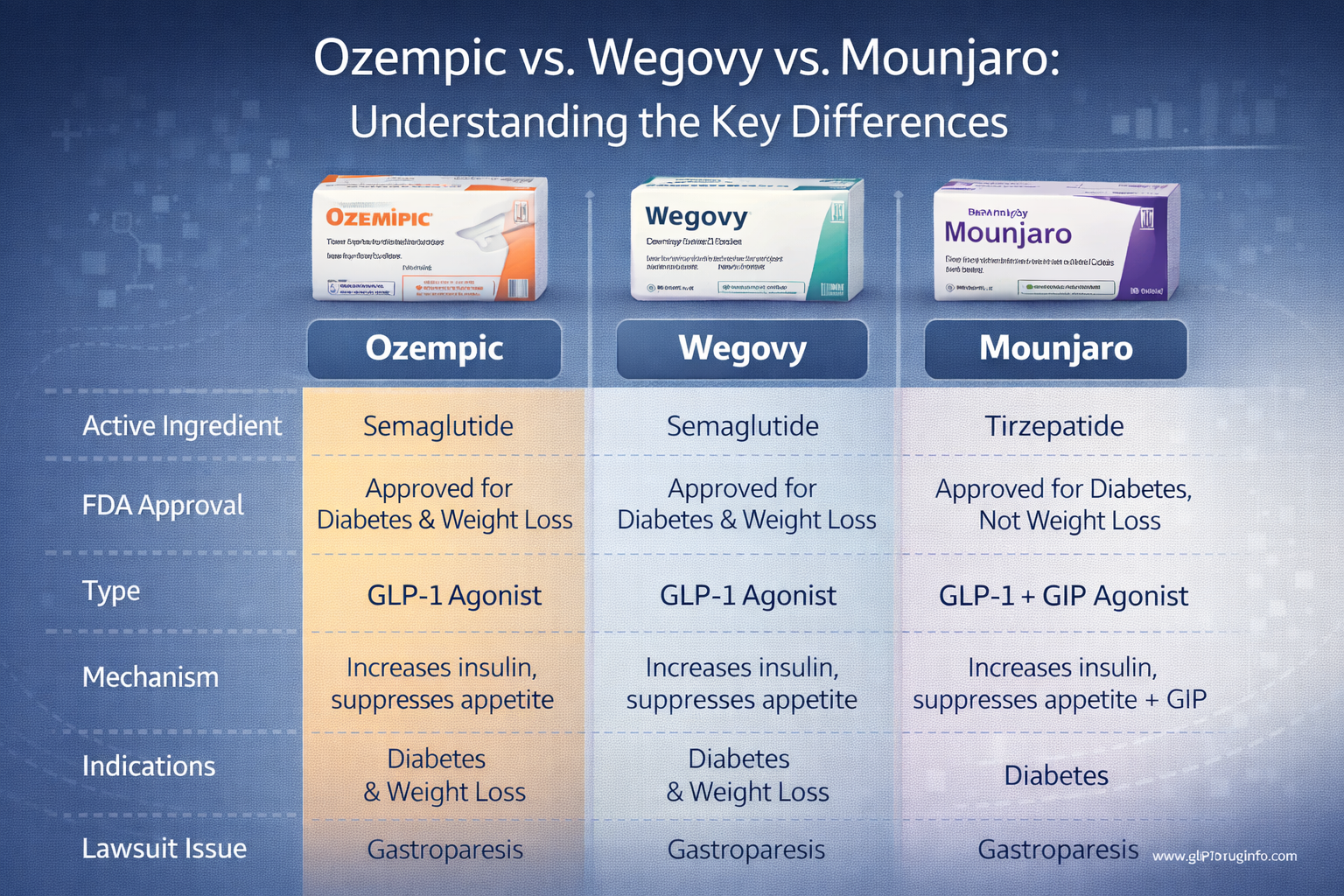
GLP-1 medications — including Ozempic, Wegovy, Rybelsus, and Mounjaro — are among the most widely used drugs in the U.S. today. While millions rely on them for diabetes and weight loss, growing reports of unexpected vision damage have raised new medical and legal concerns.
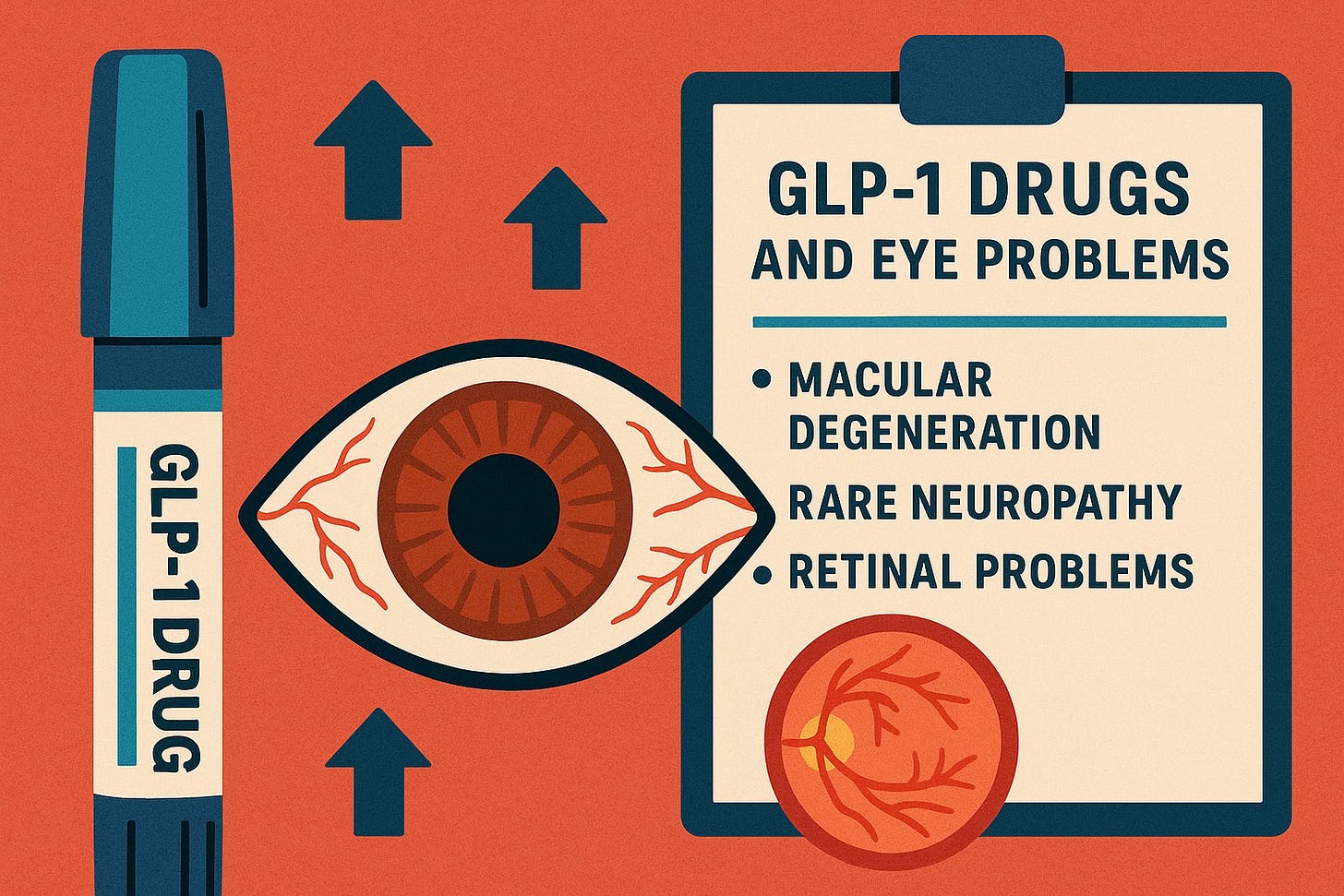
One rare but serious condition now at the center of national litigation is NAION (Non-Arteritic Anterior Ischemic Optic Neuropathy) — a form of sudden optic-nerve damage that can lead to permanent vision loss.
In recent months, NAION-related GLP-1 lawsuits have surged, prompting increased scrutiny from researchers, regulators, and patient-safety advocates.
This article explains the risks, symptoms, and legal updates patients should be aware of.
What Is NAION?
NAION occurs when blood flow to the optic nerve is suddenly reduced, causing acute damage. It is one of the most common causes of sudden optic neuropathy in adults over 50.
Typical symptoms include:
- sudden or overnight vision loss
- dimming or blurring of central vision
- reduced color perception
- vision loss usually in one eye (but can affect both)
While NAION has known risk factors such as diabetes, sleep apnea, and vascular disease, new concerns focus on whether GLP-1 drugs may increase susceptibility.
Why Are GLP-1 Drugs Being Linked to NAION?
Recent case reports and lawsuits highlight several troubling patterns:
- sudden vision loss shortly after starting Ozempic, Wegovy, or Mounjaro
- NAION occurring in patients without traditional risk factors
- mounting legal claims alleging insufficient warning labels
Researchers are investigating possible mechanisms, including:
- rapid blood pressure changes
- dehydration from nausea/vomiting
- metabolic shifts affecting optic-nerve blood flow
While evidence is still emerging, the increasing volume of reports has triggered significant concern.
Legal Landscape: NAION Lawsuits and MDL Requests
As of 2025, attorneys across the country are filing GLP-1 NAION lawsuits, alleging that drug manufacturers failed to warn about the risk of severe vision loss.
Common allegations include:
- failure to disclose the potential link to NAION
- inadequate warnings despite growing case reports
- marketing that overstated benefits while minimizing risks
Several firms have requested the creation of a federal MDL (multidistrict litigation) to consolidate NAION-related claims involving Ozempic, Wegovy, and Mounjaro.
If an MDL is approved, patients may benefit from:
- faster and more efficient case processing
- unified expert testimony
- standardized evidence review
- increased potential for settlements
Warning Signs: Symptoms Patients Should Watch For
Anyone taking a GLP-1 medication should seek immediate medical attention for:
- sudden blurry or dim vision
- loss of central vision, especially upon waking
- gray or shadow-like areas in one eye
- reduced color perception
- partial or total vision loss
NAION-related vision damage can become permanent within hours — early intervention is critical.
Who May Be at Higher Risk?
Patients with these conditions may have elevated vulnerability:
- diabetes (Type 1 or Type 2)
- sleep apnea
- uncontrolled high blood pressure
- vascular or microvascular disease
- anatomically “crowded” optic disc
Because GLP-1 drugs are frequently prescribed to people who already carry these risk factors, medical experts recommend closer eye monitoring.
What Patients Should Do Now
If you're taking Ozempic, Wegovy, Mounjaro, or Rybelsus:
1. Monitor for any sudden vision changes
NAION can progress quickly.
2. Stay hydrated
GLP-1 drugs often cause nausea/vomiting, increasing dehydration risk.
3. Discuss vision risks with your doctor
Especially if you have known optic-nerve or vascular risk factors.
4. Avoid compounded or gray-market GLP-1 products
These unregulated versions may increase risk and lack proper safety oversight.
5. Document all symptoms
This helps both medical evaluation and potential legal claims.
What’s Next? Research, Regulation, and Patient Rights
Regulators and researchers are actively studying the potential connection between GLP-1 medications and NAION. Whether this results in updated warning labels or new prescribing guidelines remains to be seen.
What is clear today:
Patients deserve transparent safety information — and legal accountability when preventable harm occurs.
If you or a loved one experienced sudden vision loss after using Ozempic, Wegovy, Mounjaro, or Rybelsus, you may qualify for a legal review.