Can GLP-1 Drugs Reduce Addictive Behaviors?
What Patients Should Know About This Emerging — and Controversial — Trend
GLP-1 medications like Ozempic, Wegovy, and Mounjaro have reshaped weight-loss treatment. But today, a new and controversial question is rising:
Can GLP-1 drugs reduce cravings, addictive behaviors, or compulsive habits?
Early reports from patients and clinicians suggest these medications may influence alcohol use, nicotine dependence, opioid cravings, and certain behavioral addictions. However — none of these uses are FDA-approved, and experts warn that the science is still in very early stages.
This article reviews the latest signals, the risks, and the legal implications for patients considering GLP-1 drugs for off-label addiction use.
Early Observations: Why People Are Talking About Addiction and GLP-1 Drugs
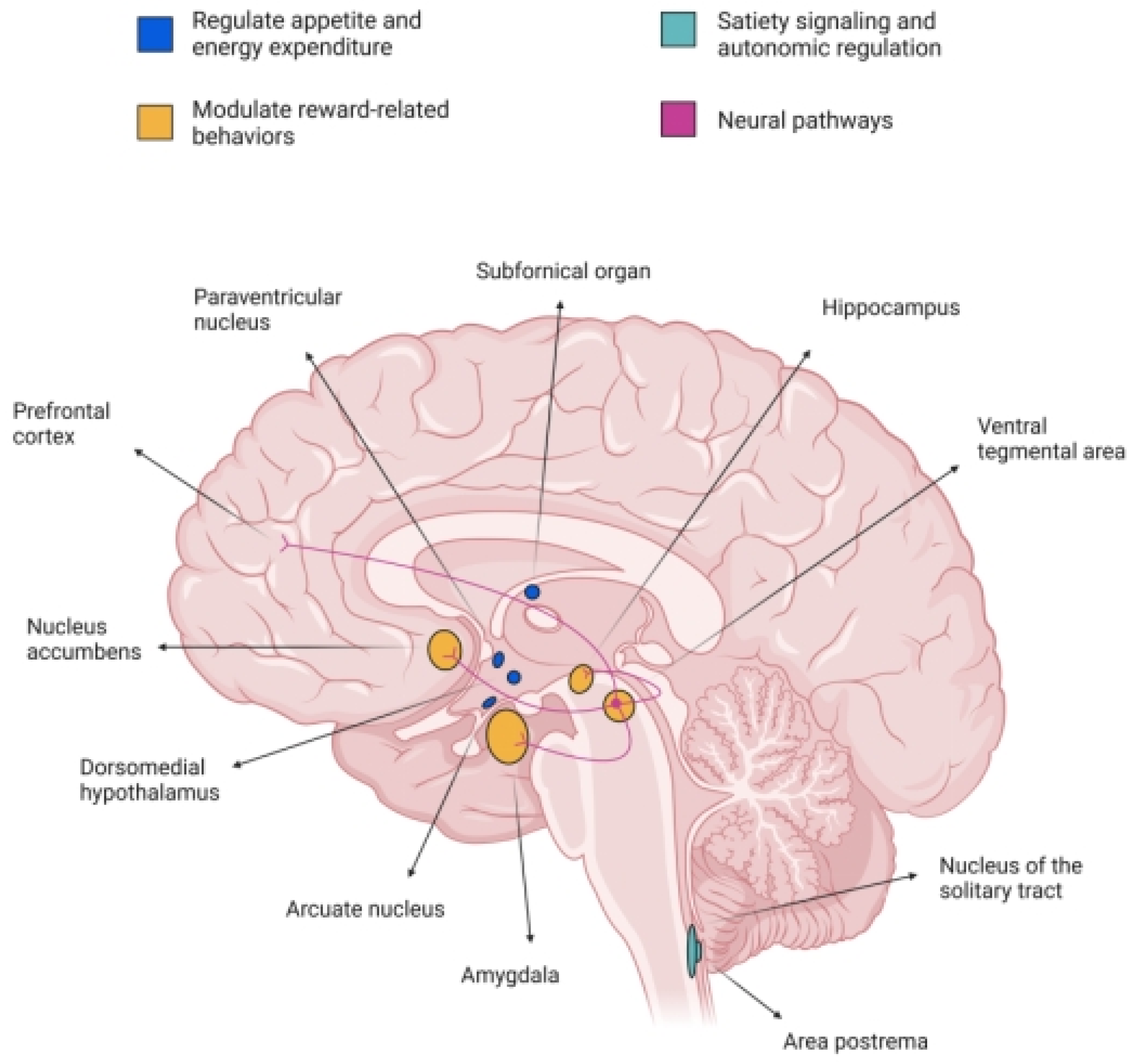
GLP-1 drugs influence not only metabolism but also reward pathways in the brain, affecting:
- dopamine signaling
- impulse control
- craving intensity
Some physicians report:
- Reduced desire for alcohol
- Lower cigarette or vaping cravings
- Less interest in binge eating
- Decreased urges in gambling or compulsive shopping
These effects are anecdotal, not proven. No GLP-1 medication is approved for addiction treatment.
Important Safety Warnings for Patients
Before considering GLP-1 drugs for addiction-related behaviors, patients should understand:
They are not approved for treating addiction.
Off-label use carries significant medical and legal risks.
Unregulated or compounded semaglutide/tirzepatide may be unsafe.
Potential side effects include:
- severe nausea/vomiting
- dehydration
- pancreatitis
- gallbladder issues
- intestinal blockage
Patients have reported long-term GI injuries — some of which are now at the center of GLP-1 lawsuits nationwide.
Legal Concerns: Why This Matters for GLP-1 Lawsuits
As interest grows in off-label addiction use, several legal questions become critical:
1. Manufacturer Liability
Drug makers may argue they are not responsible for harms caused by off-label use — but widespread real-world patterns could shift regulatory scrutiny.
2. Off-Label Prescribing Risk
Doctors who prescribe GLP-1 drugs for addiction without adequate evidence may face malpractice exposure.
3. Insurance & Compounding Risks
Lack of coverage often pushes patients toward non-FDA-approved compounded products, which have been linked to contamination, incorrect dosages, and severe injuries — now part of multiple legal claims.
Should You Use GLP-1 Drugs for Addiction?
At this stage, major medical organizations agree:
There is not enough evidence to support GLP-1 drugs as a treatment for addiction.
Anyone exploring this off-label use should:
- consult a licensed healthcare provider
- avoid compounded or unregulated GLP-1 products
- monitor for severe side effects
- seek comprehensive addiction support beyond medication
Bottom Line: Promising Idea, High Risk, and No FDA Approval
GLP-1 medications may eventually play a role in addiction treatment — but today, the science is early, the risks are significant, and legal scrutiny is rising.
As lawsuits continue to grow around long-term gastrointestinal injuries, mislabeling, and compounded semaglutide safety violations, patients should approach any unapproved use with extreme caution.
If you or a loved one has suffered severe side effects from Ozempic, Wegovy, Mounjaro, or compounded GLP-1 products, you may qualify for legal evaluation.