The Gastric Emptying Study: Your Essential Guide to Meeting MDL 3094 Requirements
Understanding the critical role of objective testing in Ozempic and Mounjaro gastroparesis lawsuits
Critical Court Ruling: Why Objective Testing Is Now Mandatory
On August 15, 2025, U.S. District Judge Karen S. Marston issued a landmark ruling in the federal multidistrict litigation (MDL 3094) that fundamentally changed the requirements for gastroparesis claims related to GLP-1 receptor agonist medications like Ozempic, Wegovy, Mounjaro, Trulicity, and Rybelsus. This decision affects thousands of current and potential plaintiffs in the Eastern District of Pennsylvania litigation.
The court's ruling established that plaintiffs alleging drug-induced gastroparesis must have undergone objective diagnostic testing—specifically a gastric emptying study—at the time of diagnosis. Clinical diagnosis based solely on symptoms is no longer sufficient to proceed with a gastroparesis claim in this litigation.
Why the Court Required Objective Testing
The pharmaceutical defendants (Novo Nordisk and Eli Lilly) successfully argued that nausea and vomiting are already listed as known side effects on their drug labels. Judge Marston agreed that to distinguish between common, temporary side effects and permanent gastroparesis injury, plaintiffs must provide scientific, objective proof of delayed gastric emptying.
The court reviewed comprehensive medical literature and found that the medical consensus across peer-reviewed articles and diagnostic guidelines requires objective testing for all forms of gastroparesis—including drug-induced gastroparesis. The court rejected plaintiff expert testimony that relied solely on differential diagnosis without objective gastric emptying studies, finding this methodology unreliable and contrary to established medical standards.
What Is a Gastric Emptying Study?
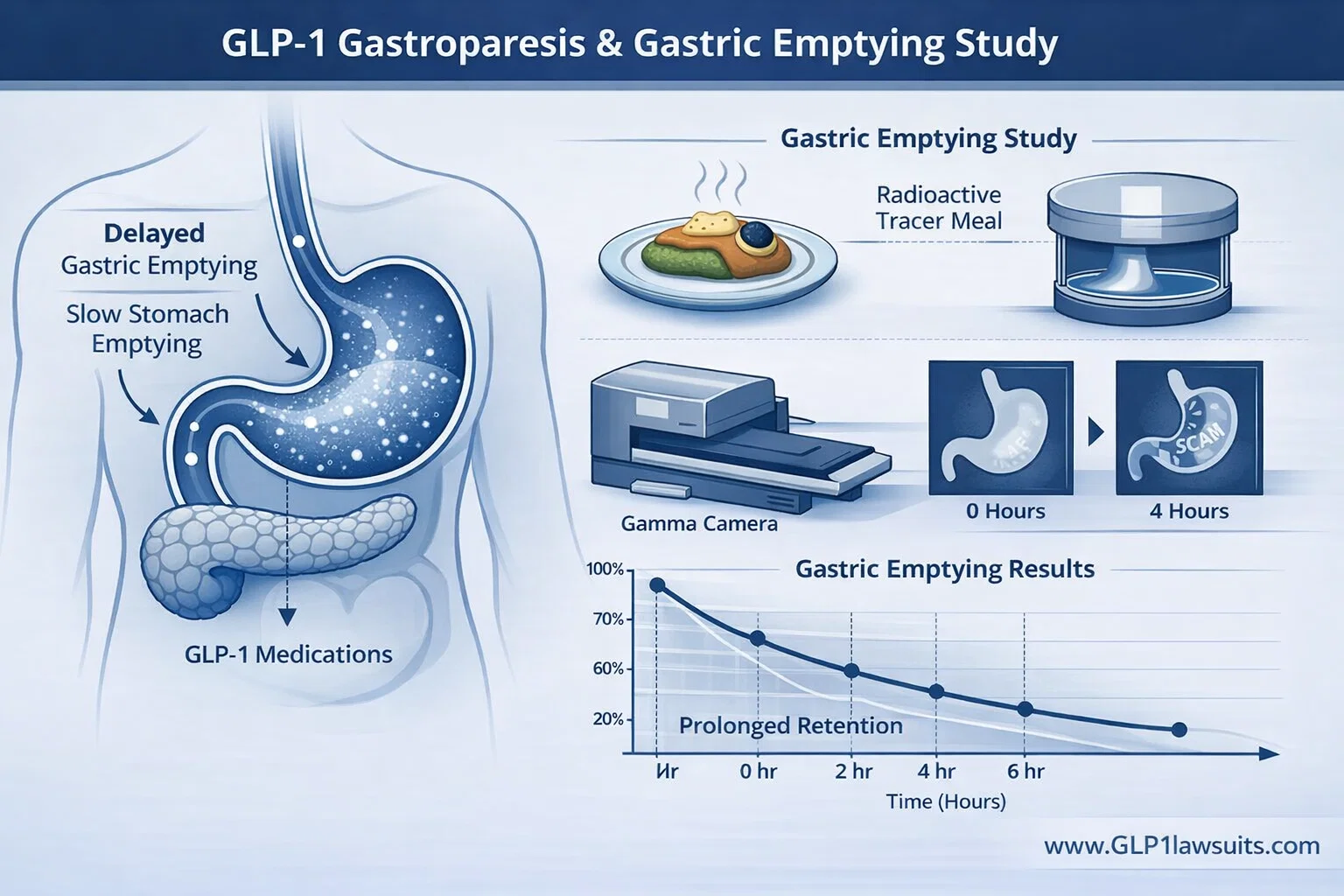
A gastric emptying study, also known as gastric emptying scintigraphy (GES), is a nuclear medicine imaging test that measures exactly how quickly food leaves your stomach. It is considered the "gold standard" diagnostic test for gastroparesis and provides objective, quantifiable data about your stomach's ability to empty properly.
How the Test Works: Step-by-Step
-
The Standardized Meal:
You consume a low-fat meal (typically scrambled egg whites, toast, jam, and water) that contains a tiny amount of radioactive tracer (Technetium-99m sulfur colloid). This radioactive material is safe, not absorbed by your body, and passes through your digestive system naturally. -
The Imaging Process:
You stand or lie in front of a gamma camera that takes pictures of your stomach at specific time intervals—immediately after eating, and then at 1 hour, 2 hours, and 4 hours after the meal. -
The Measurements:
The technician measures what percentage of the radioactive meal remains in your stomach at each time point. -
The Diagnosis:
You are diagnosed with delayed gastric emptying (gastroparesis) if more than 60% of the meal remains after 2 hours OR more than 10% remains after 4 hours. The 4-hour measurement is considered the most sensitive diagnostic timepoint.
Why the 4-Hour Test Is Essential
Medical research has consistently shown that the 4-hour gastric emptying study is significantly more sensitive than shorter tests. Studies indicate that imaging at 4 hours can detect gastroparesis in approximately 36% more patients than 1-hour imaging alone. Many patients with delayed gastric emptying will show normal results at 1 or 2 hours but will test positive at the 4-hour mark.
This is why the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine have established consensus recommendations requiring the full 4-hour protocol for standardized gastric emptying scintigraphy.
Court-Accepted Alternative Diagnostic Tests
While gastric emptying scintigraphy is the primary and most widely accepted test, Judge Marston's ruling recognizes three types of objective diagnostic testing for gastroparesis claims:
1. Gastric Emptying Scintigraphy (GES)
The 4-hour radioactive tracer meal test described above. This is the gold standard and most commonly used test. It provides the most reliable and reproducible results.
2. Gastric Emptying Breath Test (GEBT)
An FDA-approved alternative that uses stable isotope carbon-13 labeled substrates (typically spirulina or octanoic acid). You consume a meal containing the carbon-13 marker, and breath samples are collected over several hours. As your stomach empties and the meal is metabolized, carbon dioxide with the carbon-13 marker appears in your breath. This test is non-invasive, involves no radiation exposure, and can be performed in a doctor's office or even at home.
3. Wireless Motility Capsule (SmartPill)
A pill-sized wireless electronic device that you swallow. As it travels through your GI tract over approximately 5 days, it measures temperature, pH (acidity), pressure, and transit times through your stomach, small intestine, and colon. A receiver worn on your body records all the data.
Important Note:
The SmartPill was discontinued by the manufacturer in 2023, so new tests using this device cannot be performed. However, historical wireless motility capsule results from before 2023 are still valid and accepted by the court if they properly documented delayed gastric emptying.
What If You Don't Have a Gastric Emptying Study Yet?
Many patients were diagnosed with gastroparesis by their doctors based only on clinical symptoms such as nausea, vomiting, early satiety, and abdominal pain. While this "clinical diagnosis" approach is common in everyday medical practice, it is no longer sufficient for participation in the MDL 3094 litigation.
If You Are Still Experiencing Symptoms
Schedule an appointment with a gastroenterologist immediately to discuss obtaining a 4-hour gastric emptying scintigraphy study. It is critical that this test be performed while you are still symptomatic and, ideally, while you are still taking or have recently stopped taking the GLP-1 medication, as symptoms may improve over time after discontinuation.
Important Medical Considerations:
- You should have nothing to eat or drink for 4 hours before the test
- Some medications that affect gastric motility (including prokinetic drugs) must be stopped at least 48 hours before testing
- If you have diabetes, tight blood sugar control is important during testing as hyperglycemia can slow gastric emptying
- The test should not be performed if you are pregnant
- The entire test takes approximately 4 hours from start to finish
If Your Symptoms Have Improved
If you are no longer experiencing significant gastroparesis symptoms, it may be more challenging to obtain a positive gastric emptying study, as the condition may have resolved or improved after stopping the medication. However, your attorney may still be able to use other forms of objective evidence, such as:
-
Endoscopy reports documenting retained food:
If you underwent an upper endoscopy (EGD) during the period when you were symptomatic, and the gastroenterologist's report documented significant retained food in your stomach despite appropriate fasting, this may provide supporting evidence. However, the court has expressed a strong preference for the scintigraphy test over endoscopy findings alone. -
Barium studies or gastric imaging:
Other imaging studies that documented delayed gastric emptying or gastric outlet obstruction may provide additional supporting evidence.
Critical Point:
While these alternative forms of documentation may help, they are not considered equivalent to a formal gastric emptying study. Consult with an attorney immediately to discuss whether your case can proceed without a GES or whether you should attempt to obtain testing now.
Understanding the Science Phase Ruling
Judge Marston's August 2025 ruling was part of what is known as a "cross-cutting issue" in the MDL—a threshold scientific question that affects all or most cases in the litigation. The defendants had moved to exclude plaintiff expert testimony that relied on clinical diagnosis alone, and the court granted that motion under Federal Rule of Evidence 702 (the Daubert standard for expert testimony).
The court's detailed analysis reviewed:
- Comprehensive medical literature establishing that objective testing is required for diagnosing all types of gastroparesis
- Clinical practice guidelines from major gastroenterology and nuclear medicine societies
- The need to distinguish gastroparesis from functional dyspepsia, which has overlapping symptoms but different pathophysiology
- The unreliability of symptom-based diagnosis alone, given that many other conditions can cause similar gastrointestinal symptoms
While acknowledging that this requirement may exclude some plaintiffs' claims, Judge Marston emphasized that "it would be perhaps more unjust to hold Defendants potentially liable for damages based on an unreliable diagnosis."
Current Status of MDL 3094
As of December 2025, there are approximately 2,947 pending cases in MDL 3094, with hundreds of new cases being filed each month. The litigation continues to grow as more individuals come forward with claims of gastrointestinal injuries related to GLP-1 receptor agonist medications.
Eligible Injury Types
In addition to gastroparesis confirmed by objective testing, the MDL also includes claims for:
- Bowel obstruction or ileus (confirmed through imaging, surgery, or hospitalization records)
- Severe gastrointestinal injuries requiring emergency treatment or hospitalization
- Vision injuries, including Nonarteritic Anterior Ischemic Optic Neuropathy (NAION) — note that vision loss claims are being consolidated into a separate MDL 3163 but will remain under Judge Marston's oversight
Important Upcoming Dates
- Expert Discovery Deadline: March 27, 2026
- Summary Judgment Deadline: April 16, 2026
- Bellwether Trials: Expected to begin in mid-to-late 2026
Bellwether trials are test cases selected to represent common issues across the litigation. The outcomes of these trials often influence settlement negotiations and help establish the value range for claims.
Key Takeaways for Potential Plaintiffs
- Objective testing is mandatory: Clinical diagnosis based solely on symptoms is insufficient. You must have undergone a gastric emptying study (scintigraphy, breath test, or wireless capsule) that documented delayed gastric emptying.
- Act quickly if still symptomatic: If you have gastroparesis symptoms but no diagnostic testing yet, schedule a gastric emptying study with a gastroenterologist immediately.
- Gather all medical records: Obtain copies of all diagnostic tests, endoscopy reports, hospital records, prescriptions, and physician notes related to your GLP-1 medication use and gastrointestinal symptoms.
- Consult with an attorney: Given the evolving legal requirements and complex medical issues, it is essential to work with attorneys experienced in the GLP-1 litigation who can evaluate your specific situation.
- Understand the timeline: While the litigation is progressing, it may take months or years before settlement negotiations or trial outcomes provide compensation to injured plaintiffs.
Conclusion: The Path Forward
The August 2025 ruling by Judge Marston represents a significant shift in the GLP-1 gastroparesis litigation, placing objective diagnostic evidence at the center of these cases. While this requirement may create challenges for some potential plaintiffs, it also strengthens the claims of those who have proper documentation of their injuries.
If you believe you suffered gastroparesis or other serious gastrointestinal injuries from Ozempic, Wegovy, Mounjaro, Trulicity, or Rybelsus, the single most important action you can take is to obtain a formal gastric emptying study if you have not already done so. This objective diagnostic evidence has become the cornerstone of a viable gastroparesis claim in MDL 3094.
Working closely with both your medical team and experienced legal counsel will give you the best chance of successfully navigating this complex litigation and obtaining compensation for your injuries.
Contact Us for a Free Consultation
If you took Ozempic, Wegovy, Mounjaro, or Zepbound and developed gastroparesis, ileus, NAION, or DVT during the period when warnings were inadequate, you may have a legal claim.
Visit www.GLP1lawsuits.com to:
- Get a free case evaluation
- Speak with experienced attorneys
- Learn more about your legal options
- Join others seeking justice
No upfront fees. No costs unless we win your case.
Don't let the statute of limitations run out—contact us today to protect your rights and seek the compensation you deserve.
Disclaimer:
This article is for informational purposes only and does not constitute legal or medical advice. The information reflects the status of the litigation as of December 2025 and is subject to change as the case progresses. For specific guidance about your situation, Consult with an attorney and your healthcare providers. Each case is unique and past results do not guarantee future outcomes.
© 2026 GLP1lawsuits.com | All Rights Reserved