FDA Approval Timeline: When Did Drug Manufacturers Know About GLP-1 Side Effects?
Introduction
The timeline of a drug's FDA approval process is a critical component of any pharmaceutical mass tort lawsuit. In the rapidly expanding GLP-1 litigation, the central legal question is straightforward yet profound: When did Novo Nordisk, Eli Lilly, and other manufacturers know about the severe risks of side effects like gastroparesis, ileus, and NAION—and when did they update their warnings to adequately inform doctors and patients?
Understanding the chronological sequence of FDA approvals, clinical trial data, adverse event reports, and subsequent safety label changes reveals how long patients may have been taking these medications without adequate warnings about life-altering complications. This timeline is crucial because it establishes the "knowledge gap"—the period during which manufacturers knew or should have known about serious risks but failed to provide sufficient warnings.
Phase 1: The Diabetes Era (2005-2017)
The Birth of the GLP-1 Drug Class
GLP-1 receptor agonists were initially developed and approved exclusively to treat Type 2 Diabetes by helping patients control their blood sugar levels. At this stage, weight loss was observed and documented but was considered a beneficial secondary effect, not the primary therapeutic purpose.
Early FDA Approvals for Diabetes
| Brand Name | Active Ingredient | Manufacturer | Initial FDA Approval for Type 2 Diabetes |
|---|---|---|---|
| Byetta | Exenatide | AstraZeneca | April 2005 |
| Victoza | Liraglutide | Novo Nordisk | January 2010 |
| Bydureon | Exenatide (extended-release) | AstraZeneca | January 2012 |
| Trulicity | Dulaglutide | Eli Lilly | September 2014 |
| Ozempic | Semaglutide | Novo Nordisk | December 2017 |
What Was Known About Gastrointestinal Effects?
Early Clinical Trials (2005-2017): Even during the initial diabetes-focused clinical trials, researchers documented significant gastrointestinal side effects:
- Nausea and vomiting: Reported in 30-50% of trial participants
- Delayed gastric emptying: Explicitly identified as part of the mechanism of action
- Diarrhea and constipation: Common adverse events
- Abdominal pain: Frequently reported
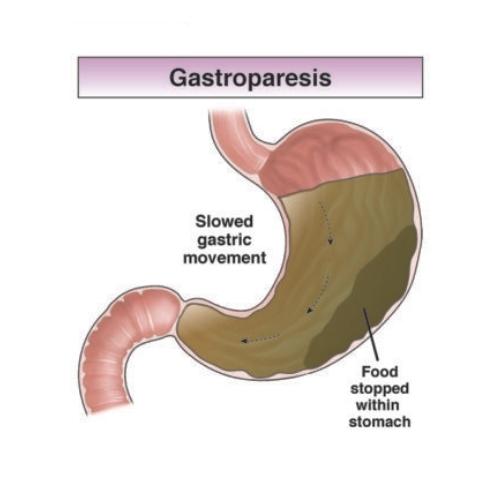
Critical Legal Point: The mechanism that slows gastric emptying—the same physiological process that can lead to gastroparesis—was present from the very beginning. This was not a hidden or unexpected effect; it was a known pharmacological action of the drug class.
Early Safety Warnings
From the outset, GLP-1 drug labels included warnings for:
- Acute pancreatitis: Inflammation of the pancreas
- Thyroid C-cell tumors: Based on animal studies showing increased risk of medullary thyroid carcinoma
- Gastrointestinal adverse reactions: Described as "generally mild to moderate" and "transient"
- Delayed gastric emptying: Noted as a mechanism that could affect oral medication absorption
What Was Missing: Despite documenting delayed gastric emptying as a known effect, early labels failed to warn about the potential for this to progress to:
- Severe, chronic gastroparesis (permanent stomach paralysis)
- Intestinal obstruction or ileus
- The need for hospitalization
- Feeding tube placement
- Long-term nutritional deficiencies
The Critical Question for This Period
Did manufacturers adequately investigate and warn about the risk of permanent gastrointestinal damage when they knew the drugs fundamentally altered stomach function?
Legal experts argue that if delayed gastric emptying was a known and expected effect, manufacturers had a duty to study and warn about the most severe manifestation of that effect—complete gastroparesis.
Phase 2: The Dedicated Weight Loss Approvals (2014-2023)
The Paradigm Shift: From Diabetes Drug to Weight Loss Blockbuster
As clinical data and real-world use demonstrated powerful weight loss effects (often 10-20% of body weight), pharmaceutical companies recognized a massive market opportunity. They began seeking FDA approval for these same drugs—often at higher doses—specifically for chronic weight management in non-diabetic patients.
FDA Approvals for Weight Loss
| Brand Name | Active Ingredient | Manufacturer | FDA Approved for Chronic Weight Management | Key Details |
|---|---|---|---|---|
| Saxenda | Liraglutide (3.0 mg daily) | Novo Nordisk | December 2014 | Higher dose than Victoza (1.8 mg for diabetes) |
| Wegovy | Semaglutide (2.4 mg weekly) | Novo Nordisk | June 2021 | Higher dose than Ozempic (0.5-2.0 mg for diabetes) |
| Zepbound | Tirzepatide (up to 15 mg weekly) | Eli Lilly | November 2023 | Dual GLP-1/GIP receptor agonist |
The Wegovy Approval: A Turning Point
June 4, 2021: The FDA approved Wegovy (semaglutide 2.4 mg) for chronic weight management in adults with obesity or overweight with at least one weight-related condition. This approval officially launched the modern GLP-1 weight loss phenomenon.
Clinical Trial Data (STEP Trials):
- STEP 1 trial: Average weight loss of 14.9% over 68 weeks
- Gastrointestinal adverse events were the most common reasons for discontinuation
- Nausea reported in approximately 44% of participants
- Vomiting in 24% of participants
- Diarrhea in 30% of participants
Critical Legal Issue: Despite these high rates of severe GI symptoms, the Wegovy label at approval described these effects as "usually transient" and did not adequately warn about:
- The potential for symptoms to become chronic or permanent
- The risk of gastroparesis requiring hospitalization
- The possibility of needing surgical intervention (feeding tubes, gastric stimulators)
Off-Label Use: The Hidden Market (2018-2021)
Important Context: Before Wegovy's official approval in June 2021, hundreds of thousands of patients were already using Ozempic off-label for weight loss. This practice was:
- Not FDA-approved for this indication
- Often prescribed by weight loss clinics and aesthetic practices
- Fueled by celebrity endorsements and social media trends
- Creating drug shortages for diabetes patients who needed it
Legal Significance: These off-label users were taking a diabetes medication at diabetes doses for weight loss purposes, potentially without full understanding of the risks. The manufacturer marketed Ozempic as a diabetes drug while simultaneously benefiting from massive off-label sales for weight loss.
The Mounjaro and Zepbound Launch
Mounjaro (Tirzepatide):
- FDA approved for Type 2 Diabetes: May 13, 2022
- Dual GLP-1/GIP receptor agonist (more potent than semaglutide)
- Showed even greater weight loss in clinical trials (SURMOUNT studies)
Zepbound (Tirzepatide):
- FDA approved for Weight Management: November 8, 2023
- Same molecule as Mounjaro, branded for weight loss indication
- Approved doses up to 15 mg weekly (higher than Mounjaro's maximum 10 mg)
Critical Timeline Note: By the time Zepbound was approved in November 2023, thousands of gastroparesis and NAION lawsuits had already been filed against GLP-1 manufacturers. Yet the approval proceeded without specific warnings about these severe complications in the initial labeling.
Phase 3: The Warnings Lag (2020-2024)
This period represents the most legally significant timeframe for the GLP-1 litigation. During these years, mounting evidence of severe gastrointestinal complications and vision loss emerged, yet official drug safety labels remained inadequate.
The Evidence Accumulates (2020-2022)
Medical Literature and Case Reports:
- 2020-2021: Gastroenterologists begin publishing case reports of severe, refractory gastroparesis in GLP-1 users
- May 2022: Case reports emerge of patients requiring hospitalization for severe vomiting and dehydration
- Throughout 2022: FDA's Adverse Event Reporting System (FAERS) database accumulates thousands of reports of serious gastrointestinal complications
What Manufacturers Knew:
Pharmaceutical companies are required to:
- Monitor adverse event databases continuously
- Review medical literature for safety signals
- Report serious adverse events to the FDA
- Update labels when new safety information emerges
Legal Allegation: Despite this accumulating evidence, manufacturers did not promptly update their labels to adequately warn about:
- The severity and potential permanence of gastroparesis
- The risk of intestinal obstruction requiring surgery
- The need for hospitalization and intensive medical management
- The possibility of needing feeding tubes or nutritional support
The Public Awareness Shift (2023)
July 2023: CNN Investigation
A major CNN health investigation brought national attention to patients suffering severe gastroparesis after taking Ozempic and Wegovy. The report featured:
- Patients unable to eat solid food months after stopping the medication
- Cases requiring feeding tubes for survival
- Physicians surprised by the severity they were observing
- Questions about whether patients were adequately warned
August 2023: Lawsuit Filings Accelerate
Following the media coverage, lawsuit filings increased dramatically:
- Hundreds of plaintiffs came forward with similar stories
- Law firms across the country began investigating claims
- More gastroenterologists spoke publicly about the pattern they were seeing
October 2023: JPML Petition Filed
Attorneys petitioned the U.S. Judicial Panel on Multidistrict Litigation to consolidate the growing number of federal lawsuits into a single MDL for coordinated pretrial proceedings.
December 2023: Initial Label Updates
Under pressure from lawsuits and media scrutiny, some label modifications began appearing, but plaintiffs argued these changes were:
- Too little, too late
- Still inadequate to convey the true severity of risks
- Buried in dense medical language rather than prominently featured
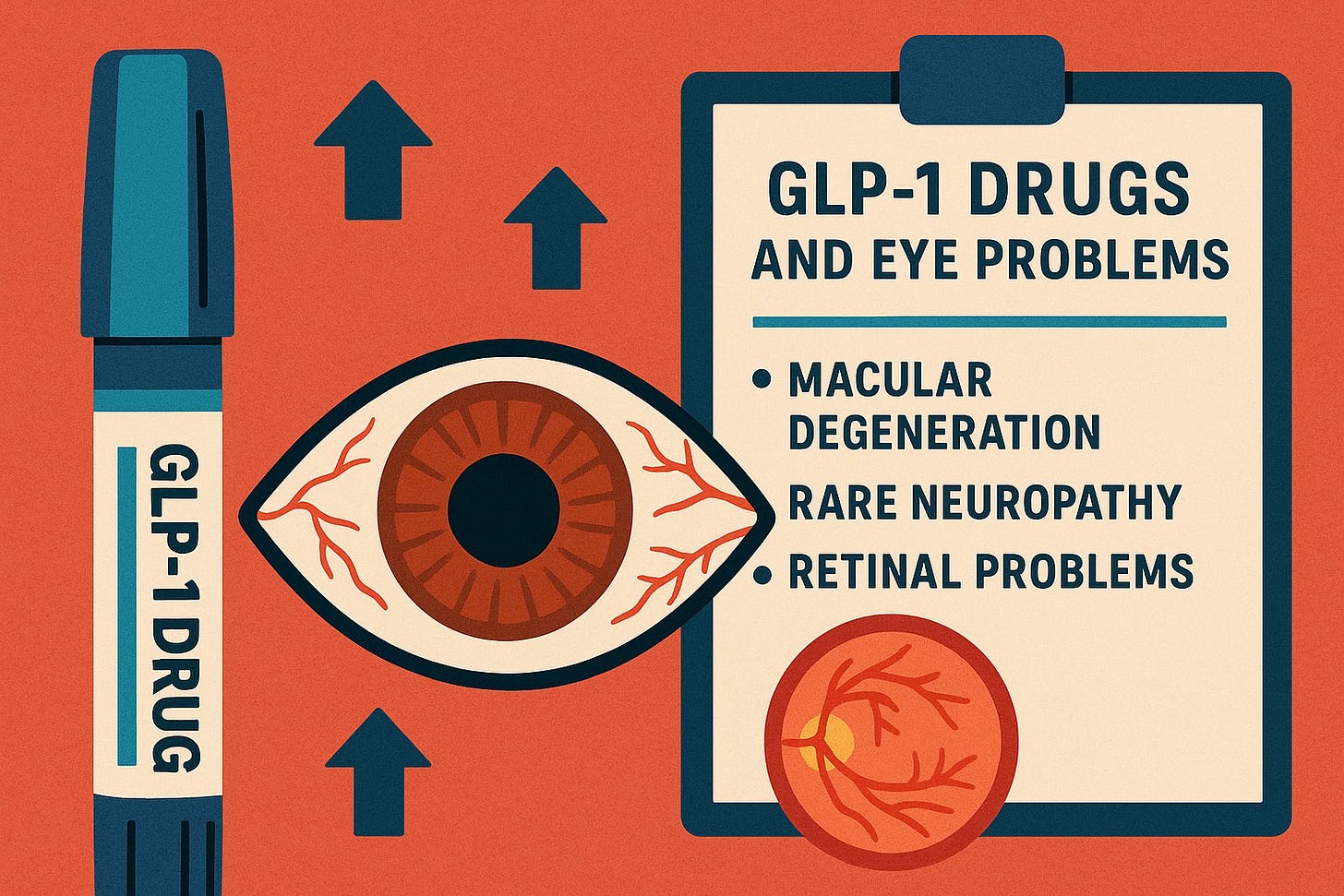
The NAION Discovery (2024)
July 2024: JAMA Ophthalmology Study
A landmark study published by Mass Eye and Ear/Harvard Medical School revealed:
- 4.3x increased risk of NAION in diabetic patients taking semaglutide
- 7.6x increased risk in patients using it for weight loss
- Sudden, permanent vision loss in previously healthy eyes
Immediate Legal Impact:
- Hundreds of new lawsuits filed specifically for NAION-related vision loss
- Questions raised about what internal company data showed about optic nerve complications
- Scrutiny of clinical trial protocols—were vision outcomes even monitored?
Label Response: As of late 2024, most GLP-1 drug labels still do not include specific warnings about NAION or sudden vision loss, despite the published evidence.
FDA Actions (2023-2024)
FDA's Role:
The FDA has taken some steps but critics argue they've been insufficient:
- September 2023: FDA acknowledges receiving reports of gastroparesis but states they are "investigating"
- March 2024: FDA adds "ileus" (intestinal blockage) to the warnings section of some GLP-1 labels
- Ongoing: FDA has not required a black box warning (the most serious warning category) for gastroparesis or NAION
Legal Criticism: Plaintiffs argue the FDA's slow response demonstrates that:
- The agency was under-resourced to monitor post-market safety adequately
- Manufacturers did not proactively report safety signals
- The drug approval process prioritized speed over comprehensive safety evaluation
The Legal Significance of the Timeline
In pharmaceutical product liability litigation, attorneys must establish three critical elements to prove a failure-to-warn claim:
1. Knowledge or "Should Have Known"
What must be proven: The manufacturer knew about the severe dangers OR should have known based on available scientific evidence, adverse event reports, and post-market surveillance.
Evidence that supports this:
- Clinical trials: Pre-approval studies documented high rates of GI adverse events
- Mechanism of action: The drugs were designed to slow gastric emptying—the exact mechanism that causes gastroparesis
- Adverse event databases: Thousands of FAERS reports of severe gastroparesis filed with the FDA
- Medical literature: Published case reports and studies describing severe complications
- Internal company documents: Discovery in litigation may reveal internal emails, safety reports, and analyses showing company awareness
Timeline Application: If Novo Nordisk conducted clinical trials in 2016-2017 showing that 44% of patients experienced nausea and 24% experienced vomiting, and the mechanism of action involved delayed gastric emptying, the company should have investigated whether these effects could become severe or permanent.
2. Failure to Adequately Warn
What must be proven: Despite having knowledge of the risks, the manufacturer failed to provide adequate warnings to doctors and patients through drug labels, prescribing information, and marketing materials.
Evidence of inadequate warnings:
- Minimization language: Labels described GI effects as "usually mild to moderate" and "generally transient"
- Omission of severity: Labels did not warn that symptoms could be permanent, require hospitalization, or necessitate feeding tubes
- Buried warnings: Any mention of gastroparesis was hidden in dense medical terminology rather than prominently featured
- Marketing contradiction: While labels had minimal warnings, marketing materials emphasized the drugs' benefits without balanced risk discussion
Timeline Application: A patient prescribed Wegovy in 2021 would have received a label stating that nausea and vomiting were "usually transient," with no specific warning about permanent gastroparesis. If that patient developed chronic gastroparesis requiring a feeding tube, they could argue the warning was inadequate.
3. Causation: Injury Linked to Inadequate Warning
What must be proven: The inadequate warning directly led to the patient's injury—if proper warnings had been provided, the patient would not have taken the medication or would have stopped sooner, preventing the harm.
How plaintiffs establish causation:
- Patient testimony: "If my doctor had told me this could cause permanent stomach paralysis, I never would have taken it for weight loss."
- Physician testimony: "Had the label clearly warned about gastroparesis risk, I would not have prescribed this medication to my patient."
- Timeline evidence: Showing the injury occurred during the period when warnings were inadequate
- Medical causation: Expert testimony linking the drug exposure to the specific injury
Timeline Application: If a patient took Ozempic from 2019-2022 for weight loss (off-label) and developed gastroparesis in 2022, their attorney would argue:
- The patient was never warned about gastroparesis risk
- If adequately warned, they would not have used a diabetes drug off-label for weight loss
- The inadequate warning directly caused their injury by allowing uninformed use
The "Knowledge Gap": The Heart of the Litigation
The most damaging legal evidence comes from proving a knowledge gap—the period between when the manufacturer knew (or should have known) about a risk and when they adequately warned about it.
Key Knowledge Gap Periods
For Gastroparesis (2018-2023):
What they knew: Delayed gastric emptying was a known mechanism; high rates of GI adverse events documented in trials
What they didn't warn about: The potential for permanent, debilitating gastroparesis requiring medical intervention
Duration of gap: Approximately 5 years of inadequate warnings
Patients affected: Millions of prescriptions written during this period
For NAION (2021-2024):
What they should have known: Case reports of vision loss emerged; the 2024 study found significantly elevated risk
What they didn't warn about: Risk of sudden, permanent blindness
Duration of gap: At least 3 years (and ongoing—still no adequate warnings as of late 2024)
Patients affected: Hundreds of documented NAION cases
Why the Knowledge Gap Matters
Discovery Process:
During litigation, plaintiffs' attorneys will use the discovery process to obtain:
- Internal company emails and memos discussing safety concerns
- Minutes from safety committee meetings
- Draft label revisions that were never implemented
- Communications with the FDA about adverse events
- Marketing strategies that emphasized benefits while downplaying risks
Smoking Gun Evidence:
If discovery reveals that executives or scientists discussed the gastroparesis or NAION risk internally but decided not to update labels, this becomes powerful evidence of:
- Corporate knowledge of the danger
- Conscious decision to withhold warnings
- Prioritization of profits over patient safety
- Potential grounds for punitive damages
Specific Timeline Example: A Patient's Journey
To illustrate the legal significance of this timeline, consider a hypothetical but typical patient scenario:
Sarah's Timeline:
January 2020: Sarah (age 42, BMI 32) sees her doctor about weight loss. Her doctor prescribes Ozempic off-label at 1.0 mg weekly. The label describes GI side effects as "usually mild and transient."
February 2020: Sarah experiences nausea for a few weeks but it improves. Her doctor assures her this is normal and will resolve.
March 2020 - June 2021: Sarah continues Ozempic, losing 35 pounds. She periodically has nausea and vomiting but attributes it to the medication's expected effects as her doctor described.
June 2021: Wegovy is approved for weight loss, but Sarah continues on Ozempic because it's working and her insurance covers it.
September 2021: Sarah's nausea worsens significantly. She can only eat small amounts. Her doctor increases her anti-nausea medication.
December 2021: Sarah begins vomiting daily and cannot keep food down. She stops Ozempic as instructed by her doctor.
January 2022: Despite stopping the medication, Sarah's vomiting continues. She loses 15 additional pounds—not from the drug, but from inability to eat.
March 2022: Gastroenterology workup reveals severe gastroparesis. Gastric emptying study shows only 20% of food leaves her stomach after 4 hours (normal is >90%).
June 2022: After failed medical management, Sarah receives a feeding jejunostomy tube (J-tube) to provide nutrition directly to her small intestine, bypassing her paralyzed stomach.
July 2023: Sarah sees the CNN report about other patients with the same condition. She realizes she was never warned about this possibility.
September 2023: Sarah consults an attorney and files a lawsuit against Novo Nordisk.
Legal Analysis of Sarah's Case:
Knowledge Element: By 2020, Novo Nordisk knew Ozempic delayed gastric emptying and caused high rates of nausea/vomiting. Medical literature from 2019-2020 had documented cases of severe gastroparesis.
Inadequate Warning Element: Sarah's 2020 Ozempic label described GI effects as "usually mild and transient"—it did not warn about potential permanent gastroparesis or the need for feeding tubes.
Causation Element: Sarah can testify that if she had known Ozempic could cause permanent stomach paralysis requiring a feeding tube, she would never have used a diabetes medication off-label for weight loss. Her doctor can testify he would not have prescribed it off-label if he had been aware of this severe risk.
Damages: Sarah can seek compensation for:
- Medical expenses (hospitalizations, surgery, feeding tube, ongoing care)
- Lost wages (unable to work during illness)
- Reduced quality of life (cannot eat normally, dependent on tube feeding)
- Pain and suffering (physical and emotional trauma)
- Future damages (lifetime of medical management)
The Timeline's Role: Sarah's case is strengthened because she took the medication during the "knowledge gap" period (2020-2023) when Novo Nordisk knew or should have known about gastroparesis risk but provided inadequate warnings.
Comparative Timeline: What Happened vs. What Should Have Happened
What Actually Happened
| Date | Event | Warning Status |
|---|---|---|
| 2005-2017 | Early GLP-1 drugs approved for diabetes | Labels note "delayed gastric emptying" but minimize severity |
| 2014-2021 | Weight loss indications approved, off-label use explodes | Labels unchanged; no specific gastroparesis warning |
| 2020-2022 | Case reports of severe gastroparesis accumulate | Manufacturers take no action to update labels |
| July 2023 | CNN investigation brings public attention | Still no adequate gastroparesis warning |
| Aug 2023 | Lawsuits filed in large numbers | Labels begin minor updates under pressure |
| July 2024 | NAION study published showing vision loss risk | Still no NAION warning on most labels |
What Should Have Happened (Plaintiffs' Argument)
| Date | What Should Have Occurred | Legal Standard |
|---|---|---|
| 2005-2010 | Pre-approval studies of severe GI outcomes | Duty to investigate known mechanism |
| 2010-2015 | Prominent warnings about gastroparesis risk | Duty to warn about known consequences of mechanism |
| 2016-2020 | Post-market surveillance and safety studies | Duty to monitor and update based on real-world use |
| 2020-2021 | Immediate label updates when case reports emerge | Duty to act on safety signals promptly |
| 2021-2023 | Black box warning consideration for severe GI effects | Duty to use strongest warnings for severe risks |
| 2024-Present | Specific NAION warnings after study publication | Duty to update labels based on new evidence |
The Legal Gap: The difference between these two timelines represents the failure to warn that forms the basis of the litigation.
What the Timeline Reveals About Corporate Priorities
The Financial Incentive to Delay
Market Size:
- GLP-1 drugs generated over $50 billion in global sales in 2023
- Projections estimate the market could reach $100 billion annually by 2030
- Wegovy alone generated over $4.4 billion in revenue in 2023
Stock Price Impact:
- Novo Nordisk's market capitalization increased by over $200 billion between 2021-2023
- Eli Lilly became one of the most valuable pharmaceutical companies globally
- Executive compensation was tied to these market gains
Legal Theory: Plaintiffs argue that the financial stakes were so enormous that companies had a powerful incentive to:
- Minimize safety concerns
- Delay label updates that might scare patients or doctors
- Continue aggressive marketing despite emerging safety signals
- Prioritize market share over patient safety
Internal Documents May Tell the Story
What Discovery Might Reveal: During litigation, internal company documents obtained through discovery could show:
- Email exchanges where scientists raised concerns about gastroparesis or NAION but were overruled by executives
- Safety committee minutes discussing adverse events but deciding not to update labels
- Marketing strategies explicitly designed to downplay risks
- FDA communications where the company resisted adding stronger warnings
- Financial analyses showing how label changes might impact sales
Precedent: In previous pharmaceutical litigations (Vioxx, Pradaxa, talcum powder), internal documents revealed that companies often:
- Knew about risks earlier than they publicly acknowledged
- Debated how to minimize the impact on sales
- Ghostwrote studies to downplay concerns
- Trained sales reps to deflect safety questions
State of Current Warnings (Late 2024)
As of late 2024, here's where GLP-1 drug labels stand:
Current Label Status
Gastroparesis Warnings:
- Most labels now include "gastroparesis" specifically (added 2023-2024)
- However, warnings still describe it as rare and don't emphasize permanence
- No black box warning (the most serious FDA warning category)
- Language remains relatively minimizing
NAION Warnings:
- Most labels do NOT include specific NAION warnings despite 2024 study
- Generic language about "vision changes" may be present
- No guidance for patients about risk factors or monitoring
- Manufacturers have not issued Dear Doctor letters
Ileus Warnings:
- Added to some labels in 2024
- Described as a possible serious side effect
- Limited guidance on prevention or early recognition
What Plaintiffs Argue Is Still Missing
Adequate warnings should include:
- Prominence: Risks should be in bold, in patient medication guides, and discussed in marketing
- Specificity: Exact conditions (gastroparesis, NAION, ileus) named rather than vague "GI issues"
- Severity disclosure: Clear language about permanent disability, need for feeding tubes, blindness
- Frequency data: How often these severe events occur (even if rare, patients deserve to know)
- Risk factors: Which patients are at higher risk
- Monitoring guidance: How doctors and patients should watch for early signs
- Irreversibility: Clear statement that damage may be permanent
Implications for Current and Future Patients
For Patients Currently Taking GLP-1 Medications
What This Timeline Means for You:
- You need complete information: Ask your doctor specifically about gastroparesis and NAION risks
- Watch for warning signs: Any new vision changes or persistent GI symptoms warrant immediate evaluation
- Document everything: Keep detailed records of symptoms, medications, and medical visits
- Consider risks vs. benefits: Weigh whether the benefits justify the risks given your personal health situation
- Know your legal rights: If you develop complications, you may have a claim even if you signed consent forms that didn't adequately describe risks
For Future Patients
What This Timeline Suggests About Drug Safety:
- FDA approval doesn't mean risk-free: These drugs all went through FDA approval, yet serious risks weren't adequately communicated
- Early adopters face higher risks: Being first to use a newly approved drug means less real-world safety data
- Post-market surveillance is crucial: Many risks only become apparent after millions of patients use a drug
- Corporate incentives matter: Blockbuster drugs generate such enormous profits that safety may take a back seat
- Patient advocacy is essential: It often takes patient complaints and media attention to force label changes
Conclusion: The Gap Between Knowledge and Warning
The FDA approval timeline for GLP-1 drugs reveals a troubling pattern: a persistent gap between when manufacturers knew or should have known about serious risks and when they adequately warned doctors and patients.
The Central Legal Questions
- When did they know? At what point did clinical trials, adverse event reports, and scientific literature provide sufficient evidence of gastroparesis and NAION risks?
- What did they do about it? Did manufacturers promptly investigate these signals, conduct additional studies, and update labels—or did they delay and minimize?
- Who was harmed during the gap? How many patients took these medications during the years when warnings were inadequate?
- Why did they delay? Were decisions about warnings influenced by concerns about market share and stock price?
- What should happen now? What compensation do injured patients deserve, and what regulatory changes are needed to prevent this from happening again?
The Stakes
This isn't just about one drug or one company—it's about the fundamental responsibility of pharmaceutical manufacturers to:
- Prioritize patient safety over profits
- Provide complete and accurate risk information
- Monitor post-market safety vigilantly
- Update warnings promptly when new risks emerge
The timeline demonstrates that this system failed for GLP-1 drugs. The question now is whether the legal system will hold manufacturers accountable and whether regulatory reforms will prevent similar failures in the future.
Take Action
If you took Ozempic, Wegovy, Mounjaro, or Zepbound and developed gastroparesis, ileus, or NAION during the period when warnings were inadequate, you may have a legal claim.
Visit www.GLP1lawsuits.com for:
- Free case evaluation
- Timeline-specific analysis of your situation
- Connection with experienced pharmaceutical litigation attorneys
- Information about ongoing MDL proceedings
Time is critical—statutes of limitations apply. Contact us today to protect your rights.
Disclaimer
This article provides general information and legal analysis but does not constitute legal advice. Each case is unique. Consult with a qualified attorney to evaluate your specific situation.
© 2025 GLP1lawsuits.com | All Rights Reserved